Full
FullGuide »
THIS SECTION CONSISTS OF DETAILS OF THE MAIN PESTS AND DISEASES IN FIELD BEANS FOLLOWED BY FUNGICIDE AND INSECTICIDE CHECKLISTS, AND A PHOTO SECTION WITH REFERENCE IMAGES.
Many factors can affect growth of winter and spring beans, and the section below describes the main pests and diseases which reduce yield and quality.
A wide range of localised climatic effects can influence the development of foliar diseases. In certain circumstances fungicides have been shown to be useful in controlling diseases. However, responses to treatment can only be expected if weather conditions favour disease development. Routine or prophylactic treatments do not produce an economic return year after year.
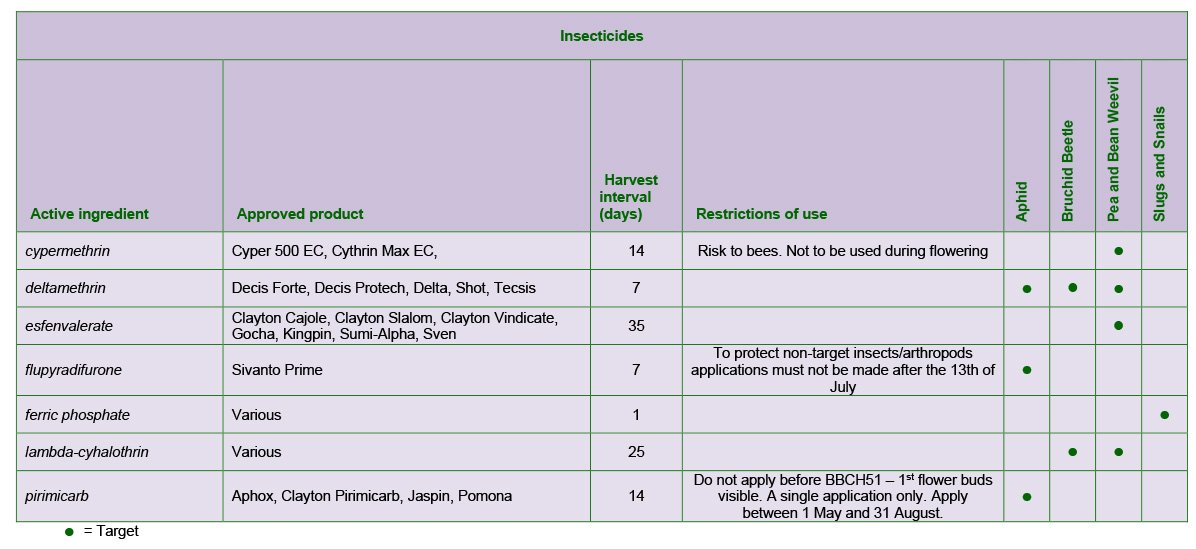
The pest can cause damage to spring beans if large numbers appear when plants are small. Leaves of attacked plants show characteristic ‘U’ shaped notches around the edges, but the main damage occurs as a result of the larvae feeding on the root nodules. Sprays may be applied at the first sign of leaf damage and repeated after 7 - 10 days.
Although winter beans are still prone to attack, they are usually too advanced in growth for the weevil or the larvae to have any appreciable effect on yield, and spray treatment is justified only when pest pressure is very high and winter beans show retarded growth.
Damage caused by the adults is visible as notching around the leaf margins. This damage does not significantly affect yield, but eggs laid during feeding produce larvae which feed in the nitrogen-fixing root nodules of peas and beans resulting in yield loss.
The adult weevils are beetle-like in shape and 4-5 mm in length. They are light grey to brown in colour with faint striping along the length of the wing cases. They have a very short ‘snout’ but have the ‘elbowed’ antennae typical of weevils.
Adults migrate from their over-wintering sites, mainly around the grassy uncultivated edges of fields previously cropped with peas or beans. Migration occurs early in the spring and this often coincides with short periods where the maximum air temperature exceeds 12°C.
During feeding, eggs laid by the female weevil are washed into the soil around the stem bases and produce larvae which begin feeding inside the root nodules. After 3-4 weeks, the larvae pupate and newly emerged adults return to the overwintering sites. There is one generation each year.
If leaf damage is anticipated, early treatment of the crop is necessary to interrupt the egg laying period and reduce root damage. A monitoring system is available which detects adults when they begin migrating in the early spring. The system was developed by Rothamsted Research and the field development was undertaken by PGRO in conjunction with Rothamsted Research and ADAS.
The monitoring system comprises five cone traps containing a pheromone lure. It can be used as an aid to decision making in the following ways:
Traps are sited on a single grassy verge or headland of a field which had been cropped with peas or beans the previous year. They should be sited by mid-February and weevils counted three times each week. Full instructions are supplied with the traps.
A threshold catch occurs when an average count per trap exceeds 30 weevils on any one recording day. Monitoring should continue until a threshold catch is reduced or until the latest sown crops have emerged (whichever is the sooner).
When a threshold has been reached, crops which have just emerged or will emerge during the next 10 days may be at risk. A spray can be applied as soon as the first sign of notching is observed if previous experience is that weevil damage occurs regularly. During periods of slow growth a second spray should be applied 10-14 days later. Crops emerging later should not be at risk. Winter-sown beans seldom require treatment. If a threshold is not reached or if it occurs more than 10 days before crop emergence there is no need to spray.
At the end of the season, the lures should be disposed of and the traps stored for re-use the following year with replacement lures. In recent years pea and bean weevil has developed partial resistance to pyrethroid active substances in some areas.
A monitoring system is available from Koppert UK to predict the likely severity of attack.
The nematode has become a major pest in field beans and can cause severe problems in wet seasons, particularly where farm-saved seed from an infested stock has been multiplied for several generations. The pest is seed-borne and can also infest soils, becoming a problem for future crops of beans. Seed should be tested for nematode, and only clean stocks should be sown.
Stem and bulb nematodes are slender, transparent and virtually microscopic. They can be found in large numbers within the stem or leaf tissue or in seed, by microscopic examination. Ditylenchus gigas is the most common and damaging species in field and broad beans, but Ditylenchus dipsaci can also be found in both. The nematodes can be seed-borne and can also survive in the soil in a free-living form. The principle routes of infestation on farms are from the use of infested seed, infested bulbs or from contaminated soil. There is no standard for infestation in the UK Field Bean Seed Certification Scheme.
Damage is often first seen as the plants reach the flowering stage. Earlier symptoms may be found at any stage after crop emergence. The plants may be stunted, and the stems thickened and twisted. Leaves become thickened and brittle with a bronze discolouration occurring in the leaf petioles. Later, the stems turn brown or rust red in colour and may swell, twist and break. Pods fail to fill evenly, and seeds are poorly developed, becoming black and shrivelled as they mature.
Affected plants may appear singly, or in larger patches of the field. The appearance of single isolated plants across the field may indicate an infested seed source, while more general patchy crop damage would indicate a pre-existing field infestation. Multiplication of nematodes is greatly enhanced during a wet spring and crop loss from this pest can be substantial. Infested seed is unsuitable for drilling, but beans are still usable in animal feed compounds and blemish-free produce may still be suitable for export.
Good practice is five years free of growing beans prior to a seed crop, and an adequate crop rotation and good weed control will, in many cases, help to prevent the pest building up in the soil. Removal and destruction of bean straw will significantly reduce the return of nematodes from the plants to the soil. Where the crop has been diagnosed as being infested, the produce should not be used for seed and a break of at least ten years should elapse before beans are grown. Best practice would be to save seed from a crop sown with certified seed and to pay attention to the crop in the field. When a crop is suspected of being infested, the harvested seed to be used for next year’s crop should be kept separate from other bulks. This seed should be sampled prior to cleaning or drying for disease, pest and germination testing.
Given that the pest can infest clean land, only tested bean seed where the pest is not detected should be used. Farm saved seed should be carefully sampled and tested before planting and purchasers of certified seed should ensure that the test has been carried out on the seed they are buying.
Before sending a seed sample for testing it is important that a representative sample is taken from the seed bulk. Preferably a sample should not represent a bulk greater than 30 tonnes for seed test results to represent the bulk.
The following guidance based on best available advice (PGRO/NIAB/SASA) will help to reduce the chance of on-farm problems:
Sampling
Seed testing
Although it is possible to detect the presence of nematodes using this guidance, due to the variability of the pest within the seed lot and between seeds, it cannot accurately determine the level of infestation or the percentage of infested seed. Therefore a ‘Clear’ test is an indication and not a guarantee that the crop or seed supplied will be free of nematodes. In addition, very low levels of infestation may be present below the limit of detection of the test, even though seedsmen take all precautions to reduce the risk of seed being infested with nematodes.
Host range and field management
Ditylenchus gigas
D. gigas is thought to be the principal species affecting field beans in the UK. It causes significant damage in field beans but has a limited host range that includes the following plant species:
|
Field beans and broad beans |
Vicia faba |
|
Lentil |
Lens culinaris |
|
Vetch |
Vicia and Lathyrus spp. |
|
Pea |
Pisum sativum |
|
Onion |
Allium spp. |
|
Corn buttercup |
Ranunculus arvensis |
|
Field bindweed |
Convolvulus arvensis |
|
White dead nettle |
Lamium album |
|
Red dead nettle |
Lamium purpureum |
|
Dead nettle |
Lamium amplexicaule |
|
Sterile oat |
Avena sterilis |
(Source: Stawniak, 2011)
Avoidance of beans in the same rotation as the species listed will help to reduce the chances of rapid build-up of D. gigas populations.
Ditylenchus dipsaci
D. dipsaci is thought to be the less common species affecting field and broad beans in the UK, but with many host plant species. A full list of species can be found at https://www.cabi.org/isc/datasheet/19287.
The species is known to affect more than 450 plant species, including Alliums, Brassicas, bulb flowers, field and broad beans, oats, sugar beet, hemp, strawberries, lucerne, tobacco, Phaseolus beans, phlox, peas, rye, potatoes, clover, maize and some weeds. As such, management of rotations is more difficult, although some crops in the rotation may be treated with nematicides, reducing populations overall.
Seed and soil can now be tested at PGRO using a molecular technique to determine the species present.
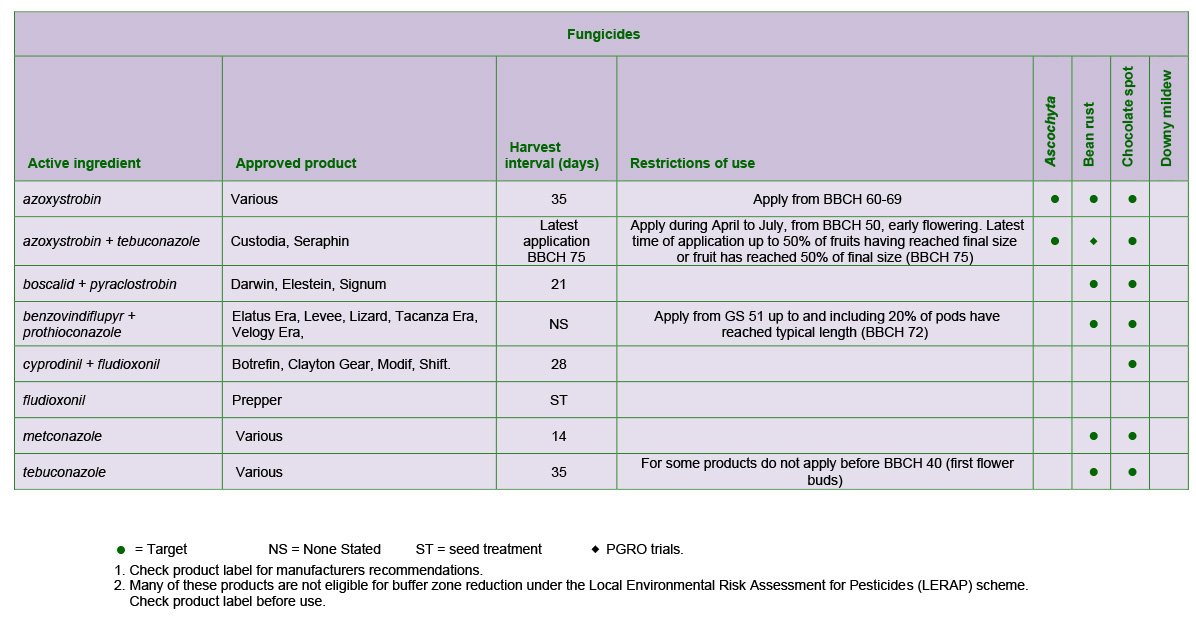
Downy mildew is prevalent on spring beans, where it causes greyish-brown, felty growth on the under-surface of the leaves. The disease is favoured by cool, humid conditions. Some varieties have resistance to the disease and ratings from 1-9 are given in the PGRO Recommended List of Varieties.
Metalaxyl M (SL567A, EAMU 0917/13) should be applied when lesions can be found on about 25% of plants and the crop has started flowering. Disease development is monitored annually and regional risk forecasts are available from Crop Monitor, https://secure.fera.defra.gov.uk/cropmonitor/.
Leaf and pod spot is primarily seed borne and the use of certified or healthy, tested seed is recommended. It is advised that farm saved seed should be tested and a seed test is available from PGRO. However, winter beans growing in close proximity to the previous year’s fields may develop symptoms during the autumn and winter from air borne spores from infected crop debris. Infection is then spread to surrounding plants by rain splashed spores.
Lesions are greyish-brown, circular to oval in shape and often develop a lighter grey centre. Unlike chocolate spot, small pin-prick sized, dark coloured fruiting bodies (pycnidia) are formed. Lesions may develop on the leaves and stems and later, on the pod surface. Here, they are sunken and become more dark in colour. Seeds inside may also be blemished. Azoxystrobin can be useful in reducing both foliar infection and seed infection in the harvested produce.
Black bean aphid can be very damaging to field beans if colonies develop prior to flowering. Spring-sown crops are usually more likely to suffer damaging attacks than winter beans. As well as forming dense, smothering colonies on the upper part of the stem, these - and the less obvious pea aphid - are able to transmit several viruses which add to the yield loss.
Dense colonies of black aphids can develop at any time on the upper part of faba bean stems from the onset of flowering. The colonies can consist of hundreds of aphids which gradually extend downwards onto the leaves and developing pods. At first, individual plants may be infested, later the aphids spread into localised patches of infested crop.
Damage to bean crops occurs mainly as the direct effect of aphids feeding. Plants fail to develop, and pods do not fill normally. The honeydew produced during feeding attracts secondary moulds as well as Botrytis spp. which cause chocolate spot. In addition, black bean aphids transmit viruses such as bean leaf roll virus.
Individual aphids are black, 1-2 mm in size, with very small white spots on the upper surface of the body. Winged aphids migrate to beans in early summer from their over-wintering hosts, which include the spindle bush (Euonymus europaeus). Each adult is viviparous and can produce several wingless nymphs each day. Colonies develop rapidly, particularly in warm, humid conditions.
Black bean aphids are relatively easy to control by aphicide sprays, but because the beans are often flowering, the choice of aphicides is limited to those which will not harm pollinating insects. Colonies first develop on headlands, but where a general infestation of 10% of plants are colonised, spraying should be carried out as soon as possible. Applications made at early flowering or when 5% of plants are infested reduces infections of aphid-transmitted viruses.
BLRV is aphid-transmitted and both the black bean aphid and pea aphid are vectors. Usually infection is more obvious where aphids infest the crop before flowering. The pea aphid is more likely to move into beans at this time. Control of early invading aphids is essential to prevent virus infection.
BYMV develops in beans at any time before flowering. Leaves are crinkled and may become pointed. Vein clearing can develop and the plant is slightly stunted. It is similar to PEMV but without the translucent spotting and streaking on the leaf surface. The virus is aphid transmitted and common in field beans. Infection may be on individual plants or groups of plants and, depending on severity, yield can be severely reduced. Pea aphid is the principal vector, although black bean aphid can also transmit the virus. Early control of aphids in flowering bean crops is the most effective means of preventing virus infection.
The disease is encouraged by long periods of overcast and humid weather conditions. Winter beans are more susceptible to infection, especially where plant populations are high. Spring beans can develop chocolate spot during humid conditions.
The disease develops as small, circular, chocolate coloured spots on the lower leaves. These become larger and may coalesce to form a greyer coloured lesion extending over the leaf surface. Stems and pods can also develop a covering of spots or flecks. Severe infections can result in defoliation.
Protectant fungicides should be applied at first pod if spotting is seen on the leaves. If severe spotting is seen earlier in the season, the first spray should be moved forward. A second spray should be applied 3-4 weeks later if spotting continues to develop on the upper parts of the plant. A third spray is seldom required as sprayer damage can cause more yield loss than late infection of chocolate spot.
A range of products and mixtures are approved.
Bruchid beetle can affect both winter and spring varieties. Adults emerge from the seed leaving a circular hole. The beetles do not breed in grain stores, but damaged produce may not be accepted for quality markets.
Adults fly to beans during flowering and lay eggs on developing pods. The larvae bore through the pod and into the seed, where they feed until mature. A pyrethroid insecticide approved for use during flowering should be applied at early pod set when the maximum daily temperature has reached at least 20ºC for two consecutive days. This should be repeated 7-10 days later.
The bruchid beetle is now widespread in all varieties of winter and spring field beans and broad beans in many parts of England. Damage to field beans is characterised by a circular hole in the seed where the adult beetles have emerged (Figure 2). In broad beans, the shelled beans may be blemished or infested with the immature larvae. In field beans, the most significant effect of the damage is to reduce the value of the crop for the human consumption export trade, or for seed. The presence of damage or larvae in broad beans makes them unacceptable for processing.
Damage
Many varieties of spring and some winter beans are exported to the Middle East for human consumption. The damage is apparent after the adults have emerged, but beans may also contain unemerged pupae or adults. High levels of damage or infestation are unacceptable for most export outlets. For seed purposes, damage at low levels may not be so serious, although at high levels, germination of the seed may be significantly reduced. In broad beans, the entry hole of the newly hatched larvae is visible as a small hole or cut in the seed coat of the shelled bean (Figure 3). This may darken after vining. If the beans are cut open, the immature larva can be found feeding within the seed (Figure 3). Blemished or infested beans in a load may lead to crop rejection by the processors.
Description and life cycle
There are many species of bruchid seed beetles worldwide, and Bruchus rufimanus is a species endemic in the UK. Unlike many other tropical species, it has only one generation each year and does not multiply in stored produce.
The adult beetle is 3.5 - 4.5 mm long, oval, black or dark brown in colour with small, grey flecks along the wing cases. A characteristic of bruchid beetles is that the wing cases do not extend to cover the abdomen completely and the hind pair of legs appears to be longer than the other two pairs.
The larvae are found inside seeds. They are white, segmented, fleshy grubs, 3 - 4 mm long when fully grown, have a light brown head with small legs on the forward three segments.
Adults fly to beans from their overwintering sites, e.g. hedgerows and standing trees, during April, May and June and after feeding for about two weeks on pollen, eggs are laid on the surface of developing pods. Pods which are 3 - 5 cm long are favoured for oviposition. Eggs are laid singly and are translucent, cigar-shaped, 0.5 mm long with a flattened irregular edged base and are fixed to the pod surface (Figure 4). Egg laying is often concentrated on pods set on the lower third of the plant. After a few days, the larvae hatch and bore through the pod wall into the developing seed. Development continues inside the seed where the larva feeds within a circular chamber just beneath the seed coat. In field beans, when fully grown, the larva pupates and this coincides with the time that seed is mature and dry. After pupation, the adult then bites its way through the seed coat, leaving a circular hole in the bean.
Many beetles emerge before harvest, but often emergence takes place in store, especially if there is a late harvest and the moisture content of the seed is high. Some smaller holes are made by the emergence of the wasp (Triaspis luteipes) which parasitises the beetles.
Control in store
Merchants may not accept field beans for any purpose with live beetles present. Although B. rufimanus does not re-infest stored beans, infested beans are best left on the farm until the emergence period is over, which in most years is complete by late October. If, however, this is not possible, then fumigation may be considered.
Beetles may be killed by a recommended grain storage insecticide. However, it is unlikely to control pupae or adults which have not yet emerged. Fumigation of beans intended for seed is possible.
Control in the field
Control of larvae as they hatch from the eggs is not possible because they penetrate the pod immediately beneath the egg case. A reduction of damage must be achieved by reducing egg laying. The timing of the spray is therefore critical. During flowering, crops should be examined for adults. This can be done by tapping the flowering stems into the hand or a small flat box.
Certain products can be used in field and broad beans during flowering and have been shown in trials to reduce the level of damage. In broad beans several products containing lambda-cyhalothrin are available under Extension of Authorisation for Minor Uses. To improve control, work in recent LINK (DEFRA)* and Innovate UK** research projects indicated that more reliable control of the pest was achieved by combining spray timing with maximum temperatures.
A spray should be applied when adults are found in the crop and 50% of the bean plants have developed the first pods on the lowest trusses, but only after the recorded maximum daily temperature has reached at least 20oC on two consecutive days. A second spray should be applied 7-10 days later. Insecticide should be applied in the early morning or late evening to avoid direct contact with bees.
*Sponsored by Defra through the Sustainable Arable LINK programme.
** Co-funded by Innovate UK through the New Approaches to Crop Protection programme.
The disease is characterised by numerous reddish-brown pustules on the leaves. It is more serious on spring beans and all varieties are susceptible. Most damage occurs if infection begins during flowering and pod set. Fungicides such as tebuconazole, azoxystrobin and metconazole may improve yield in either winter or spring beans, but treatment is unlikely to be worthwhile if infection begins when pod fill is complete and the crop is beginning to senesce.
This disease occasionally infects winter beans in damp autumn weather, and infections may be associated with preceding crops containing red clover.
Plants develop a watery stem rot, which can spread from plant to plant in dense stands. The related fungus, Sclerotinia sclerotiorum infects spring beans and also peas, rape, linseed, lupins and a range of field vegetables. Infection in spring beans is, however, very rare, but the risk should be borne in mind when planning rotations with other host crops.
Bean foot rot is caused by Fusarium culmorum and Fusarium solani although other Fusarium species might be involved too. These pathogens produce long lasting resting spores which can survive in soils for more than 10 years. In the presence of a host plant, the spores germinate to infect the plant. Roots and lower stems become blackened and root function is compromised, impairing water uptake. Foot rot infections are more likely when plants are stressed. Waterlogging and compaction are high risk factors. Lengthening rotations and improving soil health are the only available mitigation strategies.
This disease occasionally infects winter beans in damp autumn weather, and
1. Every effort is made to ensure this information is correct. However, materials may be withdrawn, or there may be additional recommendations.
2. PLEASE READ & FOLLOW PRODUCT LABEL RECOMMENDATIONS CAREFULLY
Products containing potassium phosphonates (phosphites) are used to manage crop performance in both field beans and combining peas. However, residues of phosphonic acid are regularly detected in crops above the maximum residue levels. A recent publication in the EFSA journal describes changes made to maximum residue levels in several crops, but legumes are not included (EFSA Journal, September 2020, https://doi.org/10.2903/j.efsa.2020.6240). As such, there remains a risk that crops will be rejected for use where phosphites have been applied, if they exceed maximum residue levels.